China's first domestic mpox vaccine approved for clinical trials
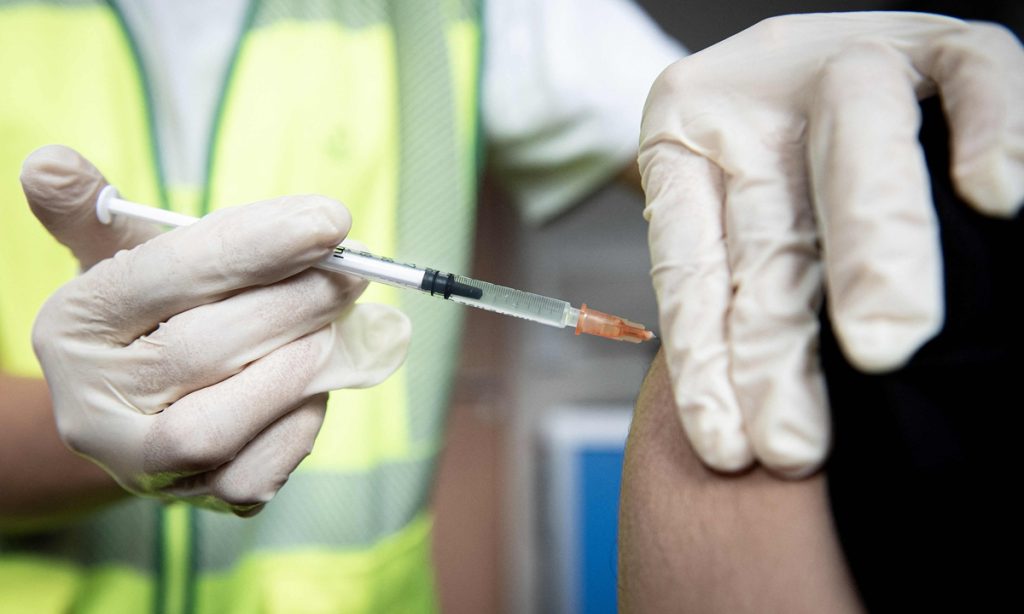
China delivered its first domestically developed mpox vaccine into clinical trials on Monday. The vaccine, independently developed by the Shanghai Institute of Biological Products, a subsidy of the China National Pharmaceutical Group (Sinopharm), is based on a live, attenuated orthopoxvirus, Modified Vaccinia Ankara (MVA), the Global Times learned from the group.
According to the institute, the MVA strain has proved its safety and efficacy as a candidate vector for vaccination. The MVA mpox vaccine is produced using a mature cell factory production process, which is stable and reliable in quality. Preclinical studies have shown its safety and its ability to generate effective immune protection against mpox virus in non-human primate models.
Between January 2022 and August 2024, more than 120 countries have reported mpox, with over 100,000 laboratory-confirmed cases reported and over 220 deaths among confirmed cases, according to data from the World Health Organization (WHO).
On August 14, the WHO declared mpox in the Democratic Republic of the Congo (DRC) and neighboring countries to be a Public Health Emergency of International Concern, the WHO's highest-level alert, along with the emergence and rapid spread of a new virus strain in DRC named clade 1b.
Lu Hongzhou, head of the Third People's Hospital of Shenzhen, told media on Monday that although the current outbreak focused on the DRC, the possibility cannot be ruled that the 1b lineage had spread globally, which requires our heightened attention.
Based on the current prevention and control measures and domestic epidemic monitoring system, the likelihood of a rapid increase in mpox infections in China remains relatively low, Lu said, while calling for the public to remain vigilant.